|
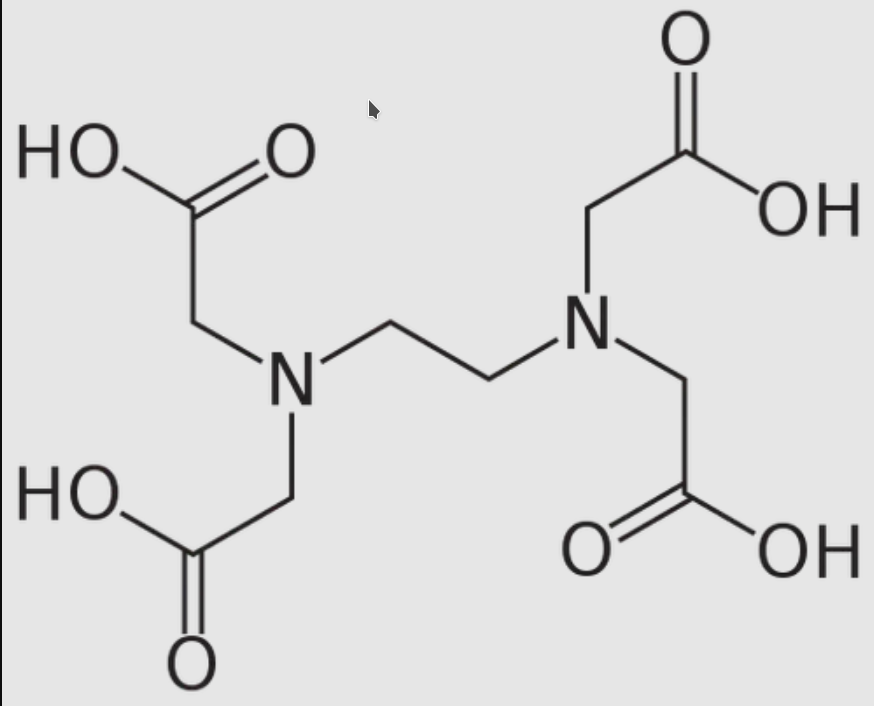
Ethylenediaminetetraacetic acid ( EDTA ), also called EDTA acid, is an aminopolycarboxylic acid with the formula [CH2N (CH2CO2H)2]2 . This white, water-insoluble solid is widely used to bind to iron (Fe2+/Fe3+ ) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH.
It is therefore used to dissolve the Fe- and Ca-containing scale and to release iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA , sodium calcium edetate, and tetrasodium EDTA, but these all function similarly.
|
  |
What is a chelated micronutrient?
The chelation process basically forms a protective shell around the respective mineral element and creates a neutral charge. This keeps them from bonding together and becoming trapped in the nutrient solution. When two molecules of the same type surround a particular mineral, it is called a chelate . However, some chelate molecules are shaped like a letter 'C' and surround the mineral with only one molecule. This type is called a 'complex'.
Types of Chelates
The chelate molecules require a bond (a type of glue) to bind them to the desired mineral element. There are a few binding agents that can be used for this, each of which has a different effect on the plants.
EDTA
One of the most common forms of chelates is ethylenediaminetetraacetic acid (EDTA). Once the elements enter the plant, this very tight bond can become a problem. When absorbed by the plant, the EDTA can form bonds with other mineral elements. EDTA can help solve one mineral deficiency, but in some cases it can cause another. EDTA has even been known to take calcium directly from the cell walls of already formed plant tissue. This causes cellular damage to the plant. In cases where a significant amount of cellular damage has occurred due to calcium loss in this way, the plant cannot maintain enough water pressure ( keyword xylem ), which can make it look as if the plants are dying of thirst (wilting).
Amino Acid Chelates
Another type of chelate is the amino acid chelate. Amino acid chelates have a slightly less strong bond than EDTA chelates. Once the mineral is absorbed by the plant and released from the amino acid, the plant can use the leftover amino acid as a nitrogen source. Amino acid chelates are also often available for use in organic nutrient formulas and come in both liquid and dry forms.
Glycine Chelates
Another form of amino acid chelates are the glycine chelates. Just like regular amino acid chelates, once the glycine is separated from the mineral element in the plant tissue, the leftover glycine (amino acid) is used by the plant tissue. The glycine amino acids have an even smaller molecular size, so they are even more easily absorbed by the plants. This makes glycine chelates especially useful in foliar applications, as they pass through the plants leaf pores ( stomata ) more easily than other, larger molecular chelates.
Summary
Amino acid chelates are very safe for plants for both root uptake and foliar applications and only become toxic to the plant when severely overdosed. In general, however, care should be taken to avoid the toxic effects of EDTA chelates. Many experts advise against using chelated minerals that use sodium as a binding agent altogether. When looking for chelated minerals, it is best to look for ones that do not use sodium. These are readily available to the plants, ones that do not promote other deficiencies (like EDTA chelates), and ones that have organic certification.
Kontext: