
Calculation of the concentrations of nutrient solutions using the following two equations
The calculation of the amount of fertilizer that has to be added to the nutrient solutions is part of a successful hydroponic production. Only multiplication, division and subtraction are used for the calculations; no advanced mathematical knowledge is required.
If you want to know more about the compositions and concentration information, the article series can be too Stoichiometry and a look at the conversion of Mol and grams When specifying the concentration of the individual elements and connections, it is helpful to better understand the complexity of the topic.
If you master the general process, producing nutrient solutions and adjusting the amount of nutrients is child's play.
Fertilizer recipes for hydroponics are almost always given in ppm (in the long form: parts per million). This may differ from the fertilizer recommendations for growing vegetables and fruits outdoors, which are generally given in lb / acre (pounds per acre).
First you have to convert ppm to mg / l (milligrams per liter) using this conversion factor: 1 ppm = 1 mg / l (1 part per million corresponds to 1 milligram per liter). For example, if 150 ppm nitrogen is required in a recipe, 150 mg / l or 150 milligrams of nitrogen in 1 liter of irrigation water are actually required.
Ppm P (phosphorus) and ppm K (potassium) are also used in recipes for nutrient solutions. This also differs from the fertilizer recommendations for growing vegetables and fruits in the field, which use P2O5 (phosphate) and K2O (potash). The fertilizers are also given as phosphate and potash. Phosphate and potash contain oxygen, which must be taken into account in hydroponic calculations. P2O5 contains 43% P and K2O contains 83% K.
Let us check the previous circumstances:
1 ppm = 1 mg / l
P2O5 = 43% P
K2O = 83% K
Nutrient solution tanks are usually measured in gal ( gallons ) in the United States. When we convert ppm to mg / l, we work with liters. To convert liters into gallons, use the conversion factor of 3.78 l = 1 gal ( 3.78 liters corresponds to 1 gallon ). The invoice is also given below for continental interested parties.
Depending on the scale you use to weigh fertilizers, it may be useful to convert milligrams into grams: 1,000 mg = 1 g ( 1,000 milligrams correspond to 1 gram ). If your scale measures in pounds, you should use this conversion: 1 lb = 454 g ( 1 pound = 454 grams ).
Let us summarize these circumstances:
3.78 l = 1 gallon
1000 mg = 1 g
454 g = 1 lb
Now we have all the necessary circumstances. Let's look at an example.
How do you determine how much 20-10-20 fertilizer is needed to deliver 150 ppm N with a 5 gallon tank and a fertilizer injector that is at a concentration of 100:1 is set?
First, write down the concentration you know you want to reach. In this case it is 150 ppm N or 150 mg N / l.

Note that we multiply by 1. This allows you to cancel the units that are the same in the numerator and denominator. Now we can paint "mg N" and get the unit g N / l water.

Continue this process by converting liters into gallons. Most containers are still traded in gallons ( 3.78 liters ). Entertaining here: the metric system was invented by the Britten. If you want a metric result, omit this calculation step.
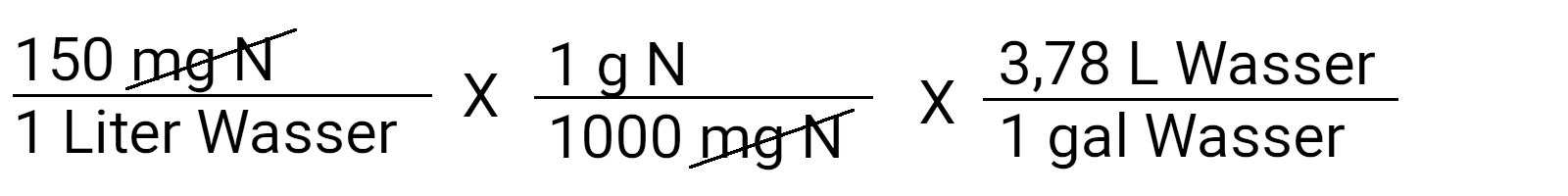
Now there are only grams of nitrogen left per gallon of water.
We'll get closer to it. Now we want to convert grams of nitrogen into grams of fertilizer. Remember that our fertilizer is a 20-10-20, which means that it contains 20% nitrogen. It can be imagined that 100 grams of fertilizer contain 20 grams of nitrogen.
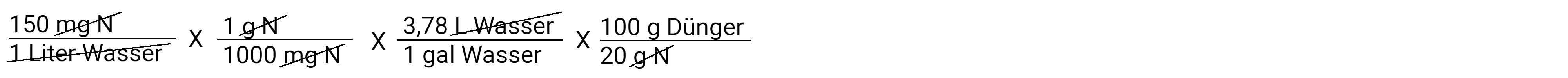
So where do we stand now? We calculated how many grams of fertilizer are needed in each gallon of irrigation water. At the moment we have a normally strong solution. Our example prompts us to calculate a concentrated solution of 100: 1. This means that for every 100 gallons of water that are applied, 1 gallon of stock solution is also applied via a fertilizer injector. We also know that our storage tank holds 5 gallons. Below see calculation for metric system (liters).
In gallons
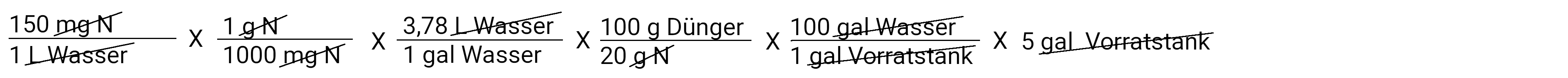
In the calculator: 150 x 1: 1000 x 3.78 x 100: 20 x 100 x 5 is 1417.5 grams on 5 gallons of water (in the storage tank)
After we have deducted everything, we have a gram of fertilizer left. This is the amount of fertilizer we need to put in our storage tank to apply 150 ppm N at a concentration of 100: 1. Multiply and divide and you get the answer 1417.5 grams of fertilizer.
In liters
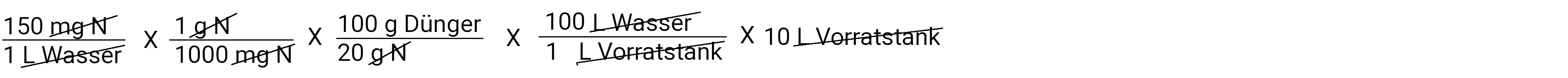
In the calculator: 150 x 1: 1000 x 100: 20 x 100 x 10 is 1500 grams per 10 liters of water ( in the storage tank )
After we have deducted everything, we have a gram of fertilizer left. This is the amount of fertilizer we need to put in our storage tank to apply 150 ppm N at a concentration of 100: 1. Multiply and divide and you get the answer 750.0 grams of fertilizer.
This means that for every 100 liters of water that is applied, 1 liter of stock solution is also applied via a fertilizer injector. We also know that our storage tank holds 10 liters.
If we measure in pounds, we have to put 0.75 kg / 1.15 lb fertilizer in our storage tank to apply 150 ppm N with a concentration of 100: 1.
You have just completed one of the two equations. Now let's look at the other one.
We just found that we need to add 750 grams of fertilizer to deliver 150 ppm nitrogen at a concentration of 100: 1. The fertilizer we used was a 20:10:20. In addition to nitrogen, we also add phosphorus and potassium. With the next equation we determine how much phosphorus we supply. This is basically the reversal of the first calculation.
We start with the amount of fertilizer that we put in our tank. The final units are ppm or mg / l. As with the previous calculation, we use our specifications until we receive these units.
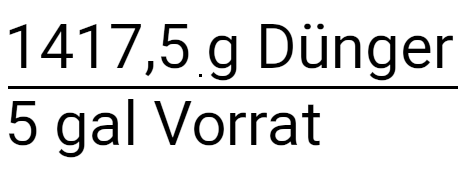
Multiply with the concentration of the nutrient solution.
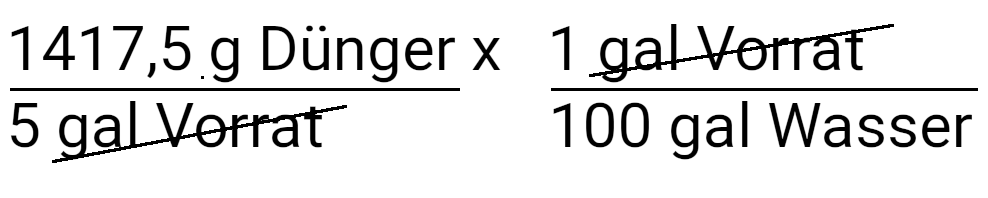
Multiply to convert to liters.
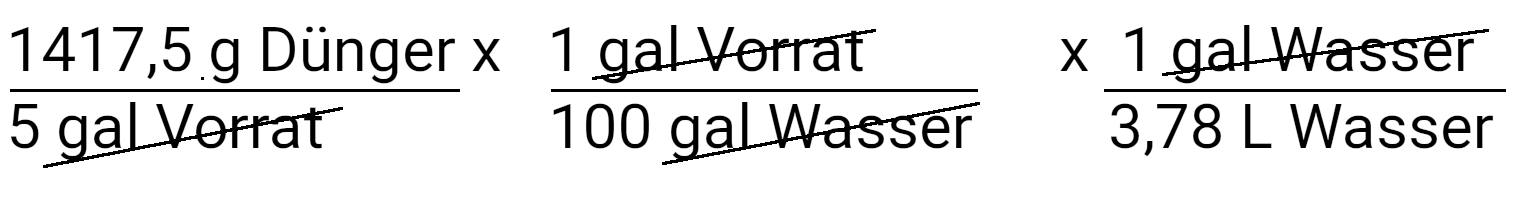
Next, convert milligrams of fertilizer into milligrams of phosphate.

Next we will convert grams of phosphate into grams of phosphorus, assuming that phosphate contains 43% phosphorus.

Finally, we convert grams of phosphorus into milligrams of phosphorus.
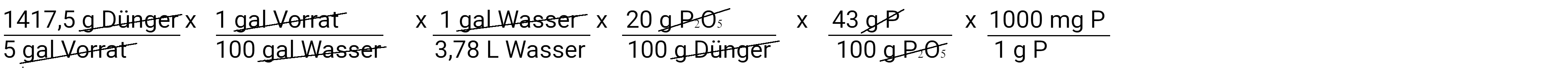
When we calculate this, we find that we have added 32.25 mg / l P or 32.25 ppm P. This is the second equation. We can also use them to determine how much potassium we have added.

We added 124.5 mg / l K or 124.5 ppm K.
With these two basic calculations, you can use any nutrient solution recipe program. How they are used to calculate a recipe can be seen in this article:
Kontext:
ID: 416



