Nutrient Solutions
 Steiner Universal Nutrient Solution is one of the most well-known hydroponic formulations and was developed by Dr. Arthur Steiner . It features a balanced ratio of macro- and micronutrients and is particularly suitable for universal applications (e.g., vegetables, herbs, fruit-bearing plants). Here is the typical composition:
Steiner Universal Nutrient Solution is one of the most well-known hydroponic formulations and was developed by Dr. Arthur Steiner . It features a balanced ratio of macro- and micronutrients and is particularly suitable for universal applications (e.g., vegetables, herbs, fruit-bearing plants). Here is the typical composition:
Steiner Universal Nutrient Solution (standard recipe)
(Figures in grams per 1000 liters of water, converted to grams per liter )
| nutrient salt | Quantity (g/L) | Nutrients supplied |
|---|---|---|
| Potassium nitrate (KNO₃) | 0.6–0.8 g/L | K (potassium), N (nitrate nitrogen) |
| Calcium nitrate (Ca(NO₃)₂) | 0.6–0.8 g/L | Ca (calcium), N (nitrate nitrogen) |
| Magnesium sulfate (MgSO₄) | 0.4–0.5 g/L | Mg (magnesium), S (sulfur) |
| Monopotassium phosphate (KH₂PO₄) | 0.2–0.3 g/L | K (potassium), P (phosphorus) |
| Iron chelate (Fe-EDTA) | 0.02–0.03 g/L | Fe (iron) |
| Trace element mix | 0.01–0.02 g/L | Mn, Zn, Cu, B, Mo (as sulfates/borates) |
Macronutrient Ratio (NPK):
- Nitrogen (N) : ~100–150 mg/L (from KNO₃ and Ca(NO₃)₂)
- Phosphorus (P) : ~30–50 mg/L (from KH₂PO₄)
- Potassium (K) : ~200–250 mg/L (from KNO₃ and KH₂PO₄)
- Calcium (Ca) : ~80–100 mg/L
- Magnesium (Mg) : ~40-50 mg/L
Micronutrients
- Iron (Fe) : 2–3 mg/L (as chelated Fe-EDTA for better availability)
- Manganese (Mn) : 0.5–1 mg/L
- Zinc (Zn) : 0.05–0.1 mg/L
- Copper (Cu) : 0.02-0.05 mg/L
- Boron (B) : 0.1–0.3 mg/L
- Molybdenum (Mo) : 0.01–0.02 mg/L
Properties of the solution
- pH value : Ideally between 5.5–6.5 (adjust with HNO₃ or KOH if necessary).
- Conductivity (EC) : ~1.5–2.5 mS/cm, depending on the plant stage.
- Advantages :
- Stabilizes nutrient absorption through high potassium and calcium levels.
- Suitable for NFT systems, Deep Water Culture (DWC) and Dutch Buckets.
Customization tips
- For fruit plants (tomatoes, cucumbers): Increase potassium (K) to 300–350 mg/L.
- For leafy vegetables (lettuce, spinach): reduce potassium and increase nitrogen.
- In case of iron deficiency : increase Fe-EDTA to 0.05 g/L.
Note on practice
Steiner's solution is often offered commercially pre-mixed (e.g., from manufacturers like Hydroponic Systems ), but many growers also mix it themselves from individual salts. Make sure the salts are of high purity (≥99%, technical grade) and always dissolve them in the correct order (calcium and magnesium salts first, then phosphates, and finally iron and trace elements).
Source: https://de.wikipedia.org/wiki/Hydrokulturd%C3%BCnger#N%C3%A4hrl%C3%B6sung_nach_Abram_Steiner
Image: https://www.pexels.com/de-de/@sasha-kim/
ID: 720
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:


Fertiliser programmes
Mixing hydroponic fertiliser yourself ?
The commercially available fertilisers consist of a complete fertiliser supplemented with macronutrients. They are offered by some hydroponics and/or fertiliser companies and vary depending on the hydroponic plant. An example of a fertiliser programme is the hydroponic tomato programme offered by Hydro-Gardens.
In this programme, growers purchase Hydro-Gardens Chem-Gro tomato formula. It has a composition of 4-18-38 and also contains magnesium and micronutrients. To make a nutrient solution, it is supplemented with calcium nitrate and magnesium sulphate, depending on the variety and/or growth stage of the plant.
Boston Public Library is licensed under CC BY 2.0
Advantages of fertiliser programmes
Very little or no mathematical calculations are required to prepare nutrient solutions.
Disadvantages of fertiliser programmes
Another disadvantage is that fertiliser programmes do not allow farmers to take into account the nutrients already present in the water source. For example, if a water source has a potassium content of 30 ppm, there is no way to adjust the amount of potassium added in the fertiliser programme. And too much potassium can in turn block the uptake of other nutrients.
Fertilizer programs can be more expensive than using
Recipes for the production of nutrient solutions.
Mix recipes for nutrient solutions / hydroponics fertilizer yourself
Modified Sonneveld recipe / herbs
| element | concentration |
| Nitrogen | 150 ppm |
| Phosphorus | 31 ppm |
| Potassium | 210 ppm |
| Calcium | 90 ppm |
| Magnesium | 24 ppm |
| Iron | 1 ppm |
| Manganese | 0.25 ppm |
| Zinc | 0.13 ppm |
| copper | 0.023 ppm |
| Molybdenum | 0.024 ppm |
| Boron | 0.16 ppm |
It is at the discretion of the breeder which fertilizers he uses to produce a nutrient solution according to a recipe. The fertilizers commonly used include:
| fertilizer | Dosage, contained nutrients |
|---|---|
| Calcium nitrate | 15.5 – 0 – 0.19% calcium |
| Ammonium nitrate | 34 – 0 – 0 |
| Potassium nitrate | 13 – 0 – 44 |
| Sequestrene 330TM | 10% iron |
| Potassium phosphate monobasic | 0 – 52 – 34 |
| Magnesium sulfate | 9.1% magnesium |
| Borax (laundry quality) | 11% boron |
| Sodium molybdate | 39% molybdenum |
| Zinc sulfate | 35.5% zinc |
| Copper sulfate | 25% copper |
| Magnesium sulfate | 31% manganese |
Advantages of nutrient solution recipes
Nutritional solutions allow fertilizers to be adjusted based on the nutrients contained in water sources. An example: A gardener uses a water source with 30 ppm potassium and produces the modified Sonneveld solution for herbs that requires 210 ppm potassium. It would have to add 180 ppm potassium ( 210 ppm - 30 ppm = 180 ppm ) to the water in order to obtain the amount of potassium required in this recipe.
With recipes, nutrients can be easily adjusted. When a leaf analysis report indicates that a plant has iron deficiency. It is easy to add more iron to the nutrient solution.
Since recipes make it easy to adapt, fertilizers can be used more efficiently than in fertilizer programs. Using recipes can be less expensive than using fertilizer programs.
Disadvantages of nutrient solution recipes
It has to be calculated how much fertilizer has to be added to the nutrient solution. (Link to performing calculations). Some people may feel intimidated by the calculations involved. However, the calculations only require uncomplicated mathematical skills based on multiplication and division.
A high-precision scale is also required for the measurement of micronutrients, since the required quantities are very small. Such a scale can be found on Amazon from 30.- €: e.g .: KUBEI 100g / 0.001g.
This is about the calculation of nutrient solutions for your own needs
Kontext:
ID: 415
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:


Calculation of the concentrations of nutrient solutions using the following two equations
The calculation of the amount of fertilizer that has to be added to the nutrient solutions is part of a successful hydroponic production. Only multiplication, division and subtraction are used for the calculations; no advanced mathematical knowledge is required.
If you want to know more about the compositions and concentration information, the article series can be too Stoichiometry and a look at the conversion of Mol and grams When specifying the concentration of the individual elements and connections, it is helpful to better understand the complexity of the topic.
If you master the general process, producing nutrient solutions and adjusting the amount of nutrients is child's play.
Fertilizer recipes for hydroponics are almost always given in ppm (in the long form: parts per million). This may differ from the fertilizer recommendations for growing vegetables and fruits outdoors, which are generally given in lb / acre (pounds per acre).
First you have to convert ppm to mg / l (milligrams per liter) using this conversion factor: 1 ppm = 1 mg / l (1 part per million corresponds to 1 milligram per liter). For example, if 150 ppm nitrogen is required in a recipe, 150 mg / l or 150 milligrams of nitrogen in 1 liter of irrigation water are actually required.
Ppm P (phosphorus) and ppm K (potassium) are also used in recipes for nutrient solutions. This also differs from the fertilizer recommendations for growing vegetables and fruits in the field, which use P2O5 (phosphate) and K2O (potash). The fertilizers are also given as phosphate and potash. Phosphate and potash contain oxygen, which must be taken into account in hydroponic calculations. P2O5 contains 43% P and K2O contains 83% K.
Let us check the previous circumstances:
1 ppm = 1 mg / l
P2O5 = 43% P
K2O = 83% K
Nutrient solution tanks are usually measured in gal ( gallons ) in the United States. When we convert ppm to mg / l, we work with liters. To convert liters into gallons, use the conversion factor of 3.78 l = 1 gal ( 3.78 liters corresponds to 1 gallon ). The invoice is also given below for continental interested parties.
Depending on the scale you use to weigh fertilizers, it may be useful to convert milligrams into grams: 1,000 mg = 1 g ( 1,000 milligrams correspond to 1 gram ). If your scale measures in pounds, you should use this conversion: 1 lb = 454 g ( 1 pound = 454 grams ).
Let us summarize these circumstances:
3.78 l = 1 gallon
1000 mg = 1 g
454 g = 1 lb
Now we have all the necessary circumstances. Let's look at an example.
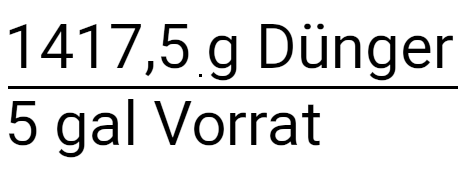
How do you determine how much 20-10-20 fertilizer is needed to deliver 150 ppm N with a 5 gallon tank and a fertilizer injector that is at a concentration of 100:1 is set?
First, write down the concentration you know you want to reach. In this case it is 150 ppm N or 150 mg N / l.

Note that we multiply by 1. This allows you to cancel the units that are the same in the numerator and denominator. Now we can paint "mg N" and get the unit g N / l water.

Continue this process by converting liters into gallons. Most containers are still traded in gallons ( 3.78 liters ). Entertaining here: the metric system was invented by the Britten. If you want a metric result, omit this calculation step.
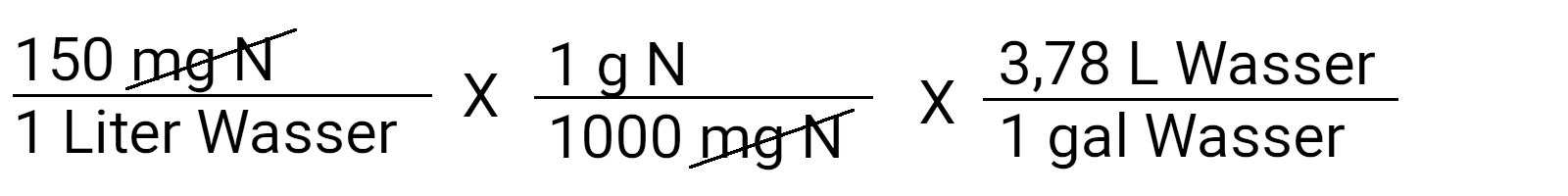
Now there are only grams of nitrogen left per gallon of water.
We'll get closer to it. Now we want to convert grams of nitrogen into grams of fertilizer. Remember that our fertilizer is a 20-10-20, which means that it contains 20% nitrogen. It can be imagined that 100 grams of fertilizer contain 20 grams of nitrogen.
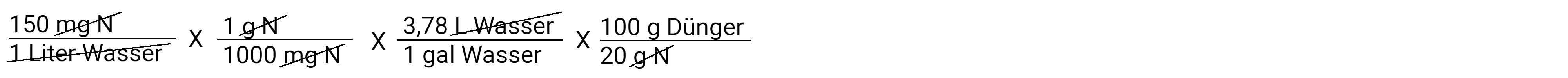
So where do we stand now? We calculated how many grams of fertilizer are needed in each gallon of irrigation water. At the moment we have a normally strong solution. Our example prompts us to calculate a concentrated solution of 100: 1. This means that for every 100 gallons of water that are applied, 1 gallon of stock solution is also applied via a fertilizer injector. We also know that our storage tank holds 5 gallons. Below see calculation for metric system (liters).
In gallons
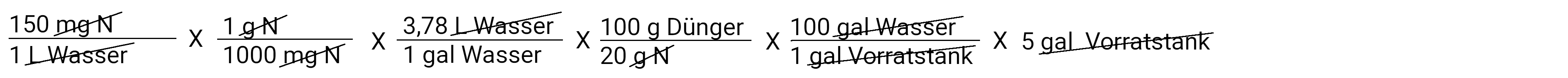
In the calculator: 150 x 1: 1000 x 3.78 x 100: 20 x 100 x 5 is 1417.5 grams on 5 gallons of water (in the storage tank)
After we have deducted everything, we have a gram of fertilizer left. This is the amount of fertilizer we need to put in our storage tank to apply 150 ppm N at a concentration of 100: 1. Multiply and divide and you get the answer 1417.5 grams of fertilizer.
In liters
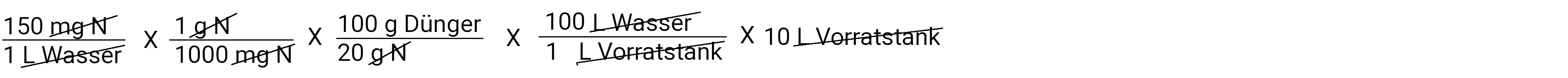
In the calculator: 150 x 1: 1000 x 100: 20 x 100 x 10 is 1500 grams per 10 liters of water ( in the storage tank )
After we have deducted everything, we have a gram of fertilizer left. This is the amount of fertilizer we need to put in our storage tank to apply 150 ppm N at a concentration of 100: 1. Multiply and divide and you get the answer 750.0 grams of fertilizer.
This means that for every 100 liters of water that is applied, 1 liter of stock solution is also applied via a fertilizer injector. We also know that our storage tank holds 10 liters.
If we measure in pounds, we have to put 0.75 kg / 1.15 lb fertilizer in our storage tank to apply 150 ppm N with a concentration of 100: 1.
You have just completed one of the two equations. Now let's look at the other one.
We just found that we need to add 750 grams of fertilizer to deliver 150 ppm nitrogen at a concentration of 100: 1. The fertilizer we used was a 20:10:20. In addition to nitrogen, we also add phosphorus and potassium. With the next equation we determine how much phosphorus we supply. This is basically the reversal of the first calculation.
We start with the amount of fertilizer that we put in our tank. The final units are ppm or mg / l. As with the previous calculation, we use our specifications until we receive these units.
Multiply with the concentration of the nutrient solution.
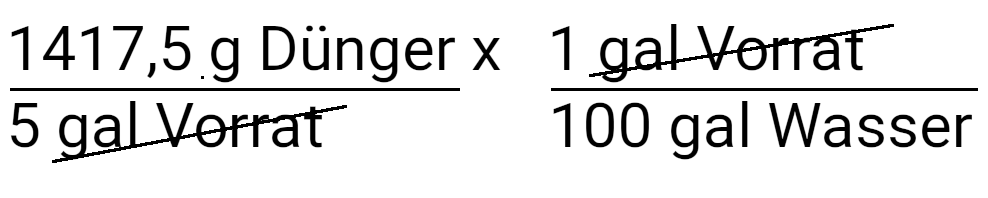
Multiply to convert to liters.
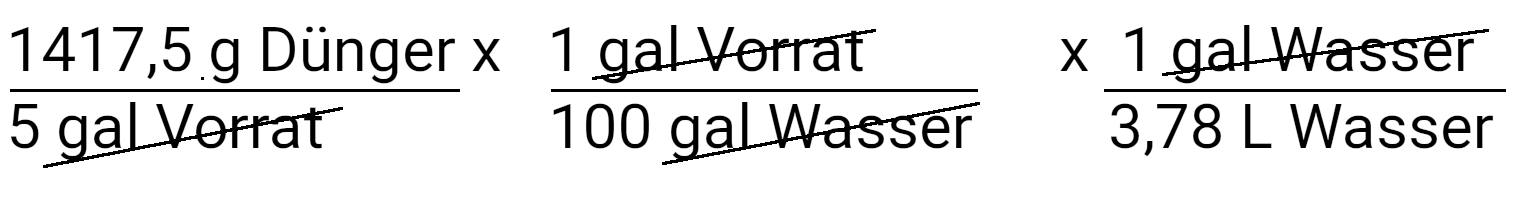
Next, convert milligrams of fertilizer into milligrams of phosphate.

Next we will convert grams of phosphate into grams of phosphorus, assuming that phosphate contains 43% phosphorus.

Finally, we convert grams of phosphorus into milligrams of phosphorus.
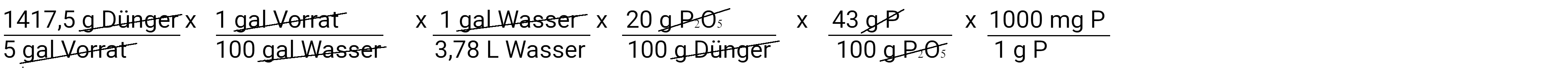
When we calculate this, we find that we have added 32.25 mg / l P or 32.25 ppm P. This is the second equation. We can also use them to determine how much potassium we have added.

We added 124.5 mg / l K or 124.5 ppm K.
With these two basic calculations, you can use any nutrient solution recipe program. How they are used to calculate a recipe can be seen in this article:
Kontext:
ID: 416
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:


|
Search in fertilizer additives/prefabricated fertilizers
|
|
Ionic balance Cation charge: – mol⁺/L Anion charge: – mol⁻/L Balance (net load): – mol/L Calculated pH (charge balance): – ¹) Estimated pH (Realistic): – ¹) Estimated electrical conductivity (EC): – mS/cm |
Composition
| Element | Source | Is: g/L | Is: mg/L = ppm | Is: mol/Liter | Is: mmol/L | Target: g/L | Target: % | Δ g/L | Name |
|---|---|---|---|---|---|---|---|---|---|
| Al | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Aluminium (Al) | |
| B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Boron (B) | |
| Be | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Beryllium (Be) | |
| C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Carbon (C) | |
| Ca | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Calcium (Ca) | |
| Cl | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Chlorine (Cl) | |
| Co | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Cobalt (Co) | |
| Cu | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Copper (Cu) | |
| F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Fluorine (F) | |
| Fe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Iron (Fe) | |
| H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hydrogen (H) | |
| K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Potassium (K) | |
| Li | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Lithium (Li) | |
| Mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Magnesium (Mg) | |
| Mn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Manganese (Mn) | |
| Mo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Molybdenum (Mo) | |
| N | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Nitrogen sum (N) | |
| NH4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Nitrogen (as NH₄⁺) | |
| NO3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Nitrogen (as NO₃⁻) | |
| Na | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Sodium (Na) | |
| O | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Oxygen (O) | |
| P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Phosphorus (P) | |
| S | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Sulphur (S) | |
| Se | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Selenium (Se) | |
| Si | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Silicon (Si) | |
| Sn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Tin (Sn) | |
| Ti | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Titanium (Ti) | |
| Zn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Zinc (Zn) |
| In CVS format for Excel, etc. | |
| Recipe / Compilation | Content substances |
¹) Regarding the pH estimate: Jones, Sonneveld & Voogt, manufacturer data sheets (Yara, Haifa, ICL), various chemical databases
¹) Regarding the EC estimate: Lide, CRC Handbook of Chemistry and Physics
²) When fertilizer mixtures (NPK + X) are used, it is not possible to calculate the EC and pH because the composition is unknown.
The values used are then estimated - and can therefore be viewed as "fantasy". When it comes to empirical values, they are marked with (real).
³) In the case of fertilizer products, some of the nitrogen is sometimes added as urea. Since this cannot be used as NO₃⁻, it is added together with the NH₄⁺ part. No further differentiation (e.g. NH₂⁻/cyanamide) is made.
⁴) Not all manufacturers reveal the chemical composition or origin of the respective NPK components.
⁵) Contain nitrogen (N) only as NH₄⁺ or NH₂⁻ or not as NO₃⁻ - but: NH₄⁺ lowers the pH in the substrate. A maximum of 5–10% of total nitrogen for hydroponics should come from NH₄⁺. More is toxic to hydroponics!
⁶) This plant growth medium is generally used for the cultivation of plant cell cultures on agar. It is also used for growing microgreens. It is listed here because there are alternative recipes that can be created here. You can find the original recipe here: Murashige - Skoog medium
⁷) Fluorine is not an essential plant nutrient element. In most cases, fluorine (vs. Fluoride F⁻) is toxic to plants because it causes photosynthetic enzymes (e.g. B. RubisCO) inhibits, damages membranes and causes oxidative stress. However, plants such as tea, aloe or some ferns can absorb significant amounts. Recommended concentrations in nutrient solutions for testing purposes are below 1 mg/L, often in the range of 0.1–0.5 mg/L. (cf. Weinstein & Davison, 2004)
ⁿ) Nitrogen (N). Origin unknown. Possible sources: e.g. NH₄⁺/ammonium, NO₃⁻/nitrate, NH₂⁻/amide, CN₂H₂/cyanamide or organic/amino acids. Without manufacturer's information for finished fertilizers.
ᵖ) Phosphorus (P). Origin unknown. Without manufacturer's information for finished fertilizers.
ᵏ) Potassium (K). Origin unknown. Without manufacturer's information for finished fertilizers.
The two most important fertilizer types when it comes to nitrogen (N):
NO₃⁻: Is immediately available to plants because nitrate is absorbed directly from the roots: For hydroponics and soil
NH₄⁺: Must be nitrified into nitrate in the soil (only possible by microorganisms): For soil and aquaponics
The NPK information on fertilizers represents the three most important plant nutrients:
* N = Nitrogen (Nitrogen)
* P = Phosphorus (Phosphorus)
* K = Potassium (Potassium)
K₂O Potassium oxide itself is not used as a fertilizer (PK/NPK fertilizer), but is used there as a unit of measurement for the proportion of potassium (e.g. B. used in the form of potassium sulfate, potassium formate, potassium nitrate or potassium chloride) in fertilizer.
These values are in Weight percent specified, and in specific chemical forms:
1. N (Nitrogen)
Chemical form: The nitrogen content is considered elemental nitrogen (N) stated, independant of the chemical compound (e.g.B. NH₄⁺, NO₃⁻, urea).
- Example: 10% N means that 10 g of pure nitrogen is contained in 100 g of fertilizer.
2. P (Phosphorus)
Chemical form: P ₂O₅ (phosphorus pentoxide) – is given non-pure phosphorus (P).
- Example: 10% P ₂O₅ means 10 g P ₂O₅ per 100 g of fertilizer.
3. K (Potassium)
Chemical form: K₂O (potassium oxide) – is also stated non-pure potassium (K).
- Example: 10% K₂O means 10 g K₂O per 100 g of fertilizer.
Example NPK information:
NPK 10-5-8 means:
- 10 % Nitrogen (N)
5 % Phosphorus Pentoxide (P ₂O₅)
8 % Potassium oxide (K₂O)
That corresponds to:
- 10 g N
5 g P₂O₅ ≈ 2,18 g P
8 g K₂O ≈ 6,64 g K
per 100g of fertilizer
Sources:
Marschner, P. (2012): Marschner's Mineral Nutrition of Higher Plants, 3rd Edition, Academic Press. -> “NPK values are expressed in oxide forms for P and K (P₂O₅ and K₂O) for historical reasons and ease of comparison.”
Mengel, K., & Kirkby, E. A. (2001): Principles of Plant Nutrition, 5th Edition, Kluwer Academic Publishers.-> “The amount of nutrient applied is usually reported in oxide equivalents, not elemental form.”
Special areas: Titanium, tin and beryllium are controversial and/or of experimental or toxicological concern. Here the literature partially contradicts itself. They are listed for completeness. No guarantee!
Lizenz: GNU GPLv3 (CopyLeft), Autor: Helmer Borgmann
³³) PS: Save this page to your computer for use without the Internet. The chart library "chart.js" for graphic display must also be on your computer: charts.js.
Adjust the path in this script so that the path points to your local computer.
For example, in Linux: <script src="/home/hirse/chart.js"><script> or Windows: <script src="C:\hier\bin\ich\chart.js"><script>
ID: 715
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

The script ( download here ) allows you to create your own fertilizer mix for hydroponics or soil from over 50 different fertilizer salts and over 200 NPK fertilizers can also be used.
Procedure
1) Select the example nutrient solution or specification and display it
Tried and tested nutrient solutions from the literature, see in the drop down menu Predefined nutrient solutions
Don't let the years fool you: practically nothing has changed since 1966, only the temperament of the discussions about it. What you should keep in mind is that for several years there have been breeds that require an extremely high dosage (EC value >= 4.0) to thrive. Select a nutrient solution here and click
In the graphic shown below, the required elements are displayed as bars. Each red bar represents 100% of an element. This graphic facilitates the following mixture of added fertilizer salts.
2) Add fertilizer salts and/or ready-made fertilizer
Select from the Drop-Down
the first additive.
Indicate the quantity in grams per litre (g/L) or milligrams per litre (ppm). Then click on
In the graphic (above) the quantities are now displayed in relation to the specifications (if you have selected one). This way you can see how exactly the quantity information corresponds to the specifications in percent.
The table also shows what amounts this corresponds to in grams, milligrams (ppm), moles and milli-moles. A column shows you the text Target And Actual value the fertilizer mixture - but only if you have chosen a specification. In the graphic at the bottom of the screen you can see the display as a long shot. Here you can better see the relationships between the individual elements.
3) Add more substances (fertilizer salts)
To add more substances click on
After each selection of another substance and quantity, click on
4) NPK fertilizer add to
The program can also take standard fertilizers into account. These usually only contain the macro-nutrients N (nitrogen) P (phosphorus) and K (potassium). With the button
<ou can add another variety of NPK fertilizer that has a different mixing ratio. This means you can combine fertilizer with 10-20-30 and 8-16-24 and see immediately whether the amount of the individual elements is too large or too small. To see the result, after entering the quantity and magnitude (gram or PPM), click on
5) Search for items in the fertilizer salts/finished fertilizer list
In the input field Search for element in fertilizer additives/prefabricated fertilizers
(e.g. Fe)
you can search for an element independently of the rest of the program. All fertilizer salts and finished fertilizers are searched for this element and displayed in a list sorted by content. Please only enter one element and click on
The element names are also under the graphic bars at the top and bottom of the page. In the table, the element name is in the left column, its common name is in the column on the right.
Intention:
This fertilizer calculator was originally only intended to add up various NPK fertilizers and calculate their total NPK at the elementary level. Since this makes little sense without comparative values, a few standard hydroponic fertilizer recipes were added to give the value a certain significance. Further refinements (Ec value, pH value) were added over time, as it is helpful to have prior knowledge of the expected conductivity (EC) and the pH value. The scope requires a different programming language, but since our customers use a wide variety of operating systems, only HTML and JavaScript remained as common denominators. Unfortunately, I'm not a fan of these "languages".
This means you can always have this "calculator" with you, even without internet. A browser (built from 2000? for very old browsers, an untested alternative is commented out) and this HTML page will suffice.
All data is stored on this page and allows you to easily expand the scope as you wish according to your own needs. If you also want the graphical representation without internet access, download the file Chart.js to your local machine and adjust the path in the script: https://borgmann-aquaponik-hydroponik.ch/chart.js... It is version 2.9.4. You can find them on the Internet here: https://cdnjs.cloudflare.com/ajax/libs/Chart.js/2.9.4/Chart.js - at least that's how it was this morning. Good luck!
PS: If there are errors or useful extensions (including fertilizer), you are worth a message write to me.
The fine print
²) When fertilizer mixtures (NPK + X) are used, it is not possible to calculate the EC and pH because the composition is unknown. The values used are then estimated - and can therefore be viewed as "fantasy". When it comes to empirical values, they are marked with (real).
⁴) The pH and EC values should be viewed with scepticism. Not always are the information provided by the manufacturers correct! - Always check with pH and EC meter -!
⚠️ Please note that some of the chemicals used can be toxic, harmful or explosive. The author assumes no responsibility for errors in the program.
ID: 705
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:


Now that you have the two basic equations for the production of nutrient solutions, we want to use them to calculate the amounts of fertilizer required for a nutrient solution recipe.
If you are not familiar with the two equations, read this first: Hydroponic systems: Calculating the concentrations of nutrient solutions using the two equations.
Here is our problem: We want to use a modified Sonneveld solution (Matson and Peters, Insidegrower) for herbs in an NFT system. We use two 5-gallon containers and injectors set to a concentration of 100: 1 and call them storage tank A and storage tank B. How much of each fertilizer do we have to put in each storage tank ?
You may be asking: why two storage tanks? This is due to the fact that certain chemicals in our fertilizer solution react with each other as soon as they come into contact with each other. In all nutrient solutions ( fertilizer mixtures ) you have calcium, phosphates and sulfates - among other things, these three chemicals for all plants vital are. The last two react with calcium and are no longer present in the form we need in our nutrient solution. They connect to each other and fall to the bottom of the container as white flakes ( precipitates ). Therefore, phosphates and sulfates must be kept separate from calcium and, when introduced into the nutrient solution of the ( system, saved from direct mixing by means of a dosing pump or measuring cup ).
Modified Sonneveld recipe for herbs
| element | concentration |
| nitrogen | 150 ppm |
| phosphorus | 31 ppm |
| potassium | 210 ppm |
| calcium | 90 ppm |
| magnesium | 24 ppm |
| iron | 1 ppm |
| manganese | 0.25 ppm |
| zinc | 0.13 ppm |
| copper | 0.023 ppm |
| Molybdenum | 0.024 ppm |
| boron | 0.16 ppm |
These are the fertilizers that we will use. Some fertilizers contain more than one nutrient in the recipe, while others contain only one. Here is a small overview Commercial fertilizer from which you can put together your recipe
| Fertilizer |
Contained nutrients
(Nitrogen phosphate potassium and other nutrients)
|
|---|---|
| Calcium nitrate | 15.5-0-0, 19% Ca (calcium) |
| Ammonium nitrate | 34-0-0 |
| Potassium nitrate | 13-0-44 |
| Potassium phosphate monobasic | 0-52-34 |
| Magnesium sulfate | 9.1% mg (magnesium) |
| Sequestrene 330 TM | 10% Fe (iron) |
| Manganese sulfate | 31% Mn (Mangan) |
| Zinc sulfate | 35.5% Zn (zinc) |
| Copper sulfate | 25% Cu (copper) |
| Boron | 11% B (Boron) |
| Sodium molybdenum | 39% Mo (molybdenum) |
The first thing you notice is that we have three sources of nitrogen (calcium nitrate, ammonium nitrate and potassium nitrate), have two sources of potassium (potassium nitrate and potassium phosphate monobasic) and one source of calcium (calcium nitrate) and phosphorus (single-base potassium phosphate). We can start calculating the calcium or phosphorus in the recipe because only one fertilizer provides each nutrient. Let's start with calcium.
The recipe provides 90 ppm calcium. We calculate how much calcium nitrate we need to use to achieve this by using the first of our two equations.
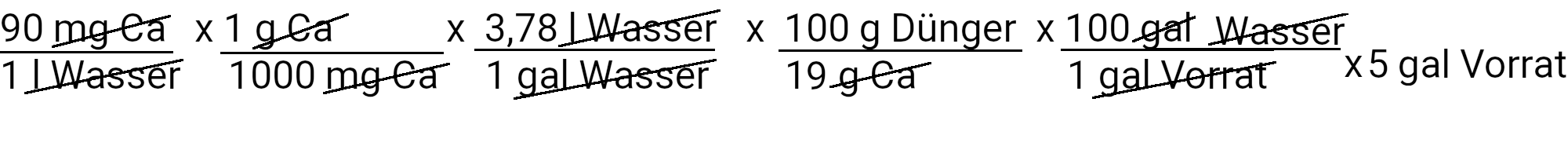
We need to add 895.3 g calcium nitrate to get 90 ppm calcium. However, calcium nitrate also contains nitrogen. We use the second equation to determine how much nitrogen should be added in ppm.
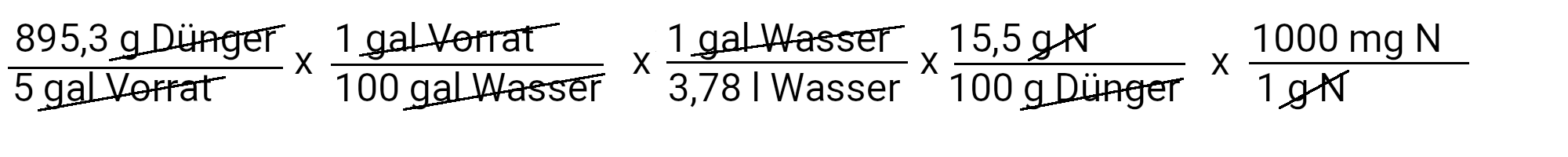
We add 73.4 mg N / l or 73.4 ppm nitrogen. Our recipe provides 150 ppm nitrogen. If we subtract 73.4 ppm nitrogen from it, we have to add 76.6 ppm nitrogen.
Let us now calculate how much single-base potassium phosphate we have to use to deliver 31 ppm phosphorus.

We need to add 262 g of potassium phosphate monobed to get 31 ppm phosphorus. However, potassium phosphate also contains single-base potassium. We use the second equation to determine how much potassium should be added in ppm.
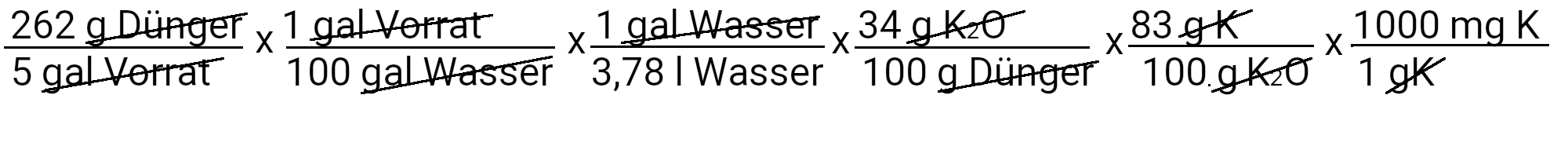
We add 39 mg K / l or 39 ppm potassium. Our recipe provides 210 ppm potassium. If we subtract 39 ppm of potassium from it, we see that we still have to add 171 ppm of potassium.
We have only one other source of potassium, namely potassium nitrate. Let's calculate how much we have to use of it.

We need to add 885 g of potassium nitrate to get 171 ppm of potassium. However, potassium nitrate also contains nitrogen. We use the second equation to determine how much nitrogen should be added in ppm.

We add 61 mg N / l or 61 ppm nitrogen. Our recipe provides 150 ppm nitrogen. We supplied 73.4 ppm nitrogen from calcium nitrate and had to add 76.6 ppm nitrogen. Now we can subtract 61 ppm nitrogen. We still have to add 15.6 ppm nitrogen. The only source of nitrogen that we have is ammonium nitrate.
Let us now calculate how much ammonium nitrate we have to use to deliver 15.6 ppm nitrogen.

We need to add 86.7 g of ammonium nitrate to get 15.6 ppm nitrogen.
At this point we have completed the nitrogen, phosphorus, potassium and calcium part of the recipe. For the other nutrients, we only need to use the first equation, since the fertilizers that we use for their supply contain only one nutrient in the recipe.
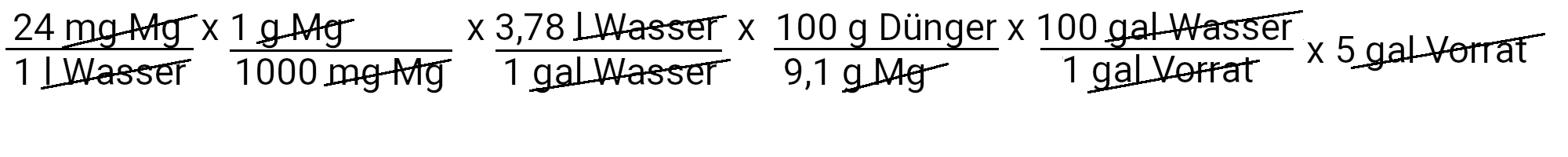
We need to add 498.5 grams of magnesium sulfate to get 24 ppm magnesium.
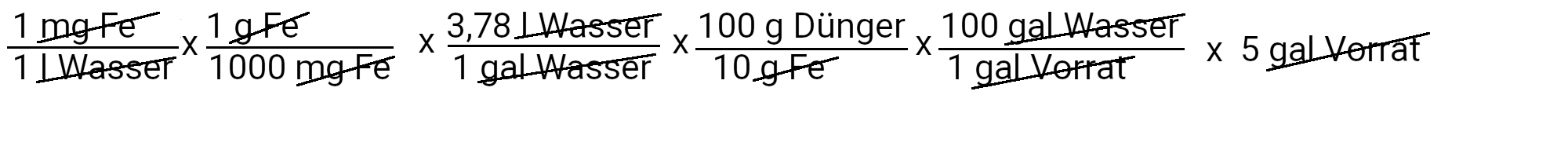 We need to add 18.9 grams of Sequestren 330 to get 1 ppm of iron.
We need to add 18.9 grams of Sequestren 330 to get 1 ppm of iron.
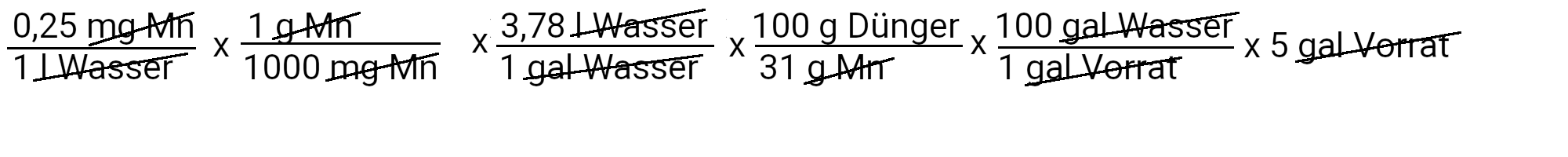
We need to add 1.5 grams of manganese sulfate to get 0.25 ppm manganese.
It is easier to weigh small amounts of fertilizers in milligrams. The conversion from milligrams to grams is therefore carried out as follows

We need to add 692 milligrams of zinc sulfate to get 0.13 ppm zinc.
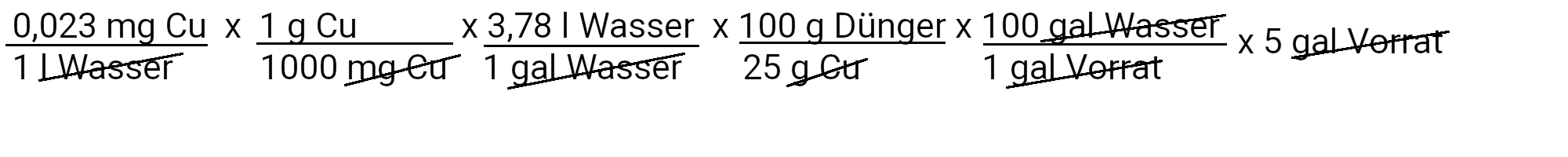
We need to add 0.17 milligrams of copper sulfate to get 0.023 ppm copper.
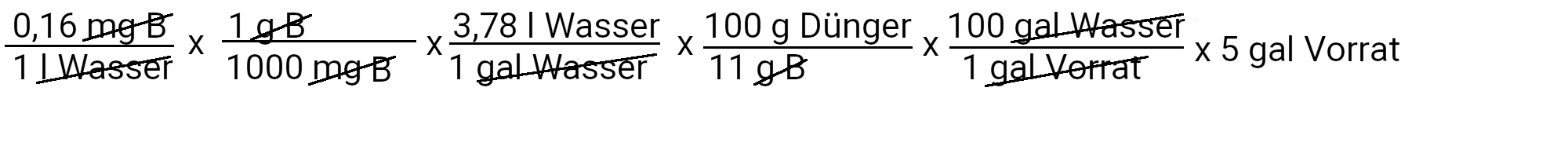
We need to add 2.8 milligrams of borax to get 0.16 ppm borax.
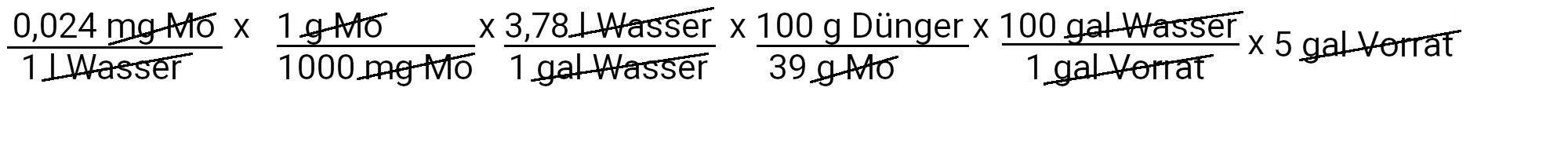
We need to add 0.12 milligrams of sodium molybdate to get 0.024 ppm molybdenum.
Summary:
| Element | Addition | Nutrient Solution |
| Calcium | 895.3 g calcium nitrate | 90 ppm calcium |
| Phosphorus | 262 g of potassium phosphate monobasic | 31 ppm phosphorus |
| Potassium | 885 g potassium nitrate | 171 ppm potassium |
| Nitrogen | 86.7 g ammonium nitrate | 15.6 ppm nitrogen |
| Magnesium | 498.5 grams of magnesium sulfate | 24 ppm magnesium |
| Iron | 18.9 grams of sequestrene 330 | 1 ppm iron |
| Manganese | 1.5 grams of manganese sulfate | 0.25 ppm manganese |
| Zinc | 692 milligrams of zinc sulfate | 0.13 ppm zinc |
| Copper | 0.17 milligrams of copper sulfate | 0.023 ppm copper |
| Boron | 2.8 milligrams of borax | 0.16 ppm boron |
| Molybdenum | 0.12 milligrams of sodium molybdate | 0.024 ppm molybdenum |
Now all calculations have been completed. Now we have to decide in which storage tank, A or B, we give the individual fertilizers. In general, the calcium should be kept in a tank other than the sulfates and phosphates, as they can form precipitates that can clog the drip bodies of the irrigation system. Using this guideline, we can put the calcium nitrate in one tank and the monobasic potassium phosphate, magnesium sulfate, manganese sulfate, zinc sulfate and copper sulfate in the other tank. The rest of the fertilizers can be placed in both tanks.
You should also consider the amount of nutrients in irrigation water. For example, if we use irrigation water that contains 10 ppm magnesium, we only need to add 14 ppm more with our fertilizer (24 ppm Mg, which are required in the recipe, minus 10 ppm Mg in water). This is a great way to use nutrients more efficiently and fine-tune your fertilizer plan.
With some micronutrients, you have to decide for yourself what you want to add. You could do a small experiment to find out whether you need to add 0.12 milligrams of sodium molybdate to your stock solution, for example, or whether you are satisfied with the performance of your plants without this addition.
One last point to consider. Sometimes the calculations don't work as well as here for fertilizers that contain more than one required nutrient, and you may need to add more of a nutrient, than is provided in the recipe to provide the other nutrient.
For example, if you apply calcium nitrate to meet calcium needs, the solution may not contain enough nitrogen. In such cases, you have to decide which nutrient you want to give priority to. For example, you could apply calcium nitrate to meet the plants' nitrogen needs because the excess amount of calcium does not harm the plants. Or you choose to apply it based on the plant's calcium needs because the lack of nitrogen is just a few ppm.
Here you will find what problems there may be with a lack and excess of fertilizer
Context:
ID: 417
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

Deficiency symptoms
Before we begin discussing the principles of plant nutrient systems in hydroponic systems, we need to define what we mean by "hydroponic."
Hydroponics is the process of growing plants in water containing nutrients. Examples of this type of hydroponic systems are NFT (Nutrient Film Technique) systems and deep water floating systems where the plant roots are placed in nutrient solutions. Another definition of hydroponics is growing plants without soil. According to this definition, growing plants in soilless media (potting soil) or other types of aggregate media such as sand, gravel, and coconut shells are considered hydroponic systems. Here we use the term hydroponics for growing plants without soil.
Essential nutrients
Plants cannot function properly without these 17 essential nutrients. These nutrients are needed to allow the processes important to plant growth and development to take place. For example, magnesium is an important component of chlorophyll. Chlorophyll (see picture) is a pigment that serves to capture light energy needed for photosynthesis. It also reflects green wavelengths and is the reason most plants are green. Magnesium is the center of the chlorophyll molecule. The table below lists the functions of the essential nutrients for plants.
Essential nutrients can be broadly divided into macronutrients and micronutrients . The classification macro (large) and micro (tiny) refers to the amounts. Both macronutrients and micronutrients are essential for the growth and development of plants. Macronutrients include carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, sulfur, calcium, and magnesium. Micronutrients include iron, manganese, zinc, boron, molybdenum, chlorine, copper, and nickel. The difference between macronutrients and micronutrients lies in the amount plants need. Macronutrients are needed in larger amounts than micronutrients. Table 1 shows the approximate content of essential nutrients in plants.
Plants obtain carbon, hydrogen and oxygen from air and water. The remaining nutrients come from the soil or, in the case of hydroponics, from nutrient solutions or aggregate media. The sources of nutrients available to plants are listed in Table 1.
Essential components of nutrient solutions, Table 1
| Nutrient (symbol) | Approximate plant content (% dry weight) |
Role in the plant |
Source of nutrients available to the plant |
| Carbon (C), hydrogen (H), oxygen (O) | 90+ % | Components of organic compounds | Carbon dioxide (CO 2 ) and water (H 2 O) |
| Nitrogen (N) | 2–4% | Component of amino acids, proteins, coenzymes, nucleic acids | Nitrate (NO3-) und Ammoniak (NH4+) |
| Sulfur (S) | 0.50% | Component of sulphur-containing amino acids, proteins, coenzyme A | Sulfate (SO4-) |
| Phosphor (P) | 0.40% | ATP, NADPMetabolic intermediates, membrane phospholipids, nucleic acids | Dihydrogenphosphat (H2PO4-), Hydrogenphosphat (HPO42-) |
| Potassium (K) | 2.00% | Enzyme activation, turgor, osmotic regulation | Potassium (K + ) |
| Calcium (Ca) | 1.50% | Enzyme activation, signal transduction, cell structure | Calcium (Ca2+) |
| Magnesium (Mg) | 0.40% | Enzyme activation, component of chlorophyll | Magnesium (Mg2+) |
| Manganese (Mn) | 0.02% | Enzyme activation, important for water splitting | Manganese (Mn 2+ ) |
| Iron (Fe) | 0.02% | Redox changes, photosynthesis, respiration | Iron (Fe 2+ ) |
| Molybdenum (Mo) | 0.00% | Redox changes, nitrate reduction | Molybdat (MoO42-) |
| Copper (Cu) | 0.00% | Redox changes, photosynthesis, respiration | Copper (Cu 2+ ) |
| Zink (Zn) | 0.00% |
Cofactor activator for enzymes
Alkohol-Dehydrogenase, Carboanhydrase
|
Zink (Zn2+) |
| Bor (Bo) | 0.01% | Membrane activity, cell division | Borat (BO3-) |
| Chlor (Cl) | 0.1–2.0% | Charge equalization, water splitting | Chlor (Cl-) |
| Nickel (Ni) | 0.000005–0.0005% | Component of some enzymes, biological nitrogen fixation, nitrogen metabolism | Nickel (Ni2+) |
| Nutrient requirement kg/ha | |
| Nitrogen | 250 |
| Phosphor | 100 |
| Potassium | 400 |
| Magnesium | 80 |
| Sulfur | 20 – 30 |
| Calcium | 60 – 80 |
| Nutrient requirement g/ha | |
| Bor | 450 – 550 |
| Manganese | 600 – 700 |
| Ferrum | 500 – 1.500 |
| Copper | 80 – 90 |
| Zinc | 250 – 350 |
PH value
This diagram shows the relationship between nutrient availability and pH value:

Graphic: Pennsylvania State University
At the bottom of the chart, various pH levels between 4.0 and 10.0 are indicated. At the top of the chart, the relative acidity or alkalinity is indicated. Within the chart, the relative nutrient availability is represented by a bar. The wider the bar, the more relatively available the nutrient is. For example, the nitrogen bar is widest at a pH of 6.0 to 7.5. This is the pH at which it is most available to plants. Between 4.0 and 4.5, it is very narrow and not as easily available to plants.
It is also important to consider the alkalinity of the water. Alkalinity is a measure of capacity. It measures the ability of the water to neutralize the acid. This is primarily due to the combined amount of carbonate (CO3) and bicarbonate (HCO3), but hydroxide, ammonium, borate, silicate and phosphate can also contribute.
When total alkalinity is low, the water has a low buffering capacity. As a result, the pH changes slightly depending on what is added to the water. When total alkalinity is high, the pH of the water is high. To lower a high pH of the water, acid can be added to the irrigation water. The amount of acid needed depends on the alkalinity of the water.
Nutrient antagonism and interactions
For example, a hydroponic tomato nutrient solution recipe calls for 190 ppm nitrogen and 205 ppm potassium. Due to an error in calculating the amount of fertilizer to use, 2,050 ppm potassium is added. An excess of potassium in the solution can cause antagonism with nitrogen (and other nutrients) and result in nitrogen deficiency even if 190 ppm nitrogen was added. The table below lists common antagonisms.
| Nutrient | Antagonist of |
|---|---|
| Nitrogen | Potassium |
| Phosphor | Zinc |
| Potassium | Nitrogen, calcium, magnesium |
| Sodium | Potassium, calcium, magnesium |
| Calcium | Magnesium, Bor |
| Magnesium | Calcium |
| Ferrum | Manganese |
| Zinc | Ion competition: high concentrations of heavy metals, copper and phosphate reduce the uptake rate of zinc: the cause of zinc deficiency in the plant does not necessarily have to be zinc-poor soil |
See also: Interactions
Problems with nutrients
Hydroponic systems are less forgiving than soil-based systems, and nutrient problems can quickly lead to plant problems. This is why nutrient solution composition and regular monitoring of the nutrient solution and plant nutrient status are critical.
The minimum law
Carl Sprengel's law of the minimum states that the growth of plants is limited by the resource that is relatively scarce (nutrients, water, light, etc.). This means that a lack of nitrogen can also lead to the plant not being able to process other nutrients. On the other hand, too much of one component can have undesirable consequences: for example, too much lime inhibits the absorption of nutrients.
Here is a brief overview of the deficiency symptoms, which can vary depending on the plant genus.
| Symptoms | N | P | K | Ca | S | Mg | Fe | Mn | B | Mo | Zn | With | Overfertilization |
| Upper leaves yellow | X | X | |||||||||||
| Middle leaves yellow | X | ||||||||||||
| Lower leaves yellow | X | X | X | X | |||||||||
| Red stems | X | X | X | ||||||||||
| Necrosis | X | X | X | X | X | ||||||||
| Points | X | ||||||||||||
| Shoots die | X | ||||||||||||
| White leaf tips | X | X | |||||||||||
| Crumpled Wheatgrass | X | X | X | ||||||||||
| Rolled yellow leaf tips | X | ||||||||||||
| Twisted growth | X |
| Damage caused by soluble salts |
Cause: Soluble salt damage can be caused by over-fertilization, poor water quality, accumulation of salts in aggregate media over time, and/or inadequate leaching. Fertilizers are salts, and in hydroponic systems they are the most common fertilizer. As water evaporates, soluble salts can build up in aggregate media if they are not adequately leached. Irrigation water can also have high levels of soluble salts, contributing to the problem.
The symptoms: Chemically induced drought can occur when the content of soluble salts in the planting substrates is too high. The result is that the plants wilt despite sufficient watering. Other symptoms include dark green foliage, dead and burned leaf edges and root death.
Detection: Soluble salt levels can be monitored/measured by tracking the electrical conductivity (EC) of irrigation water, nutrient solutions and leachate (a nutrient solution drained from the plant container).
Correction: Soluble salts can be leached out with plain water. First, determine the cause of the high soluble salts level and correct it.
| Boron toxicity | Bo |
| Calcium deficiency | Ca |
The cause: Strong temperature changes can interrupt and hinder calcium uptake. Lack of light, cold and/or too humid environmental conditions. Fertilizer level too low. Calcium deficiency can be caused by under-fertilization, a nutrient imbalance or a pH value that is too low. It is also related to moisture management, high temperatures and low air circulation. Calcium is a mobile nutrient and is transported through the plant in the water-bearing tissues. Fruits and leaves compete for water. Low relative humidity and high temperatures can lead to an increased transpiration rate and increased transport to the leaves. In this case, a calcium deficiency can develop in the fruits.
The symptoms: The apical meristems (these are the dividing tissues of the plant) are deformed and die off without any noticeable symptoms on the oldest leaves. The upper part of the stem and flower bud may bend. Small and deformed leaves on the upper side. Unusually dark green leaves. Premature flower and fruit drop. After a deficiency, the leaves that were developing at the time of the deficiency often show a typical deformation/drying out or a white edge. This is called tip burn and is particularly common in lettuce and strawberries. Browning of the inside of a stem/head, around the growing point like in celery (black heart). Typical symptoms are also blossom end rot on peppers and tomatoes. Symptoms usually first appear as brown leaf edges on new plants or on the underside of the fruit. Blossom end rot in tomatoes and peppers. As symptoms progress, you may see brown, dead spots on the leaves. A lack of sufficient calcium can lead to rot.
Detection: Leaf analysis. Fruits have a poorer shelf life.
Correction : Make sure the pH is between 5.5 and 6.5. Add calcium nitrate or calcium chloride depending on whether you need the extra nitrogen or not.
In the greenhouse: Increase the temperature. More light. Without wind, the plant's nutrient transport is reduced - ensure air movement in the greenhouse.
| Ferrum deficiency | Fe |
| Sulphur deficiency | S |
The cause: Too little or incorrectly proportioned fertilizer. A pH value that is too low also blocks the absorption of sulfur. At a pH value of 4.0, sulfur absorption stops completely. Too little magnesium.
The symptoms: Extensive yellowing of the leaf tissue and the leaf veins. Often the younger parts of the plant first and later the whole plant. Symptoms are more likely to appear in young or freshly growing leaves at the top of the plant. Sulfur is an immobile nutrient. This means that sulfur can only be re-disposed (transported) relatively slowly by the plant. Lime green to yellow discoloration on leaves is characteristic of sulfur deficiency. It starts at the leaf stalk and moves to the leaf edges and tip. As the disease progresses, the entire leaves first turn yellow, then later brown and necrotic and then die completely. Sometimes purple/reddish leaf stalks on the affected leaves or even a purple stem. The symptoms of a mild deficiency are usually limited to the top of the plant. The middle part of the plant is hardly affected, lower leaves almost never.
Detection: leaf analysis.
Correction : increase the fertilizer dose. Correct the pH: keep it well above 4.0. 5.5 to 6.5 is a good average for many plants. Enrich the soil with Epsom salt / magnesium sulfate / MgSO 4 : one teaspoon per 2 liters of water (approx. 1% concentration).
| Nitrogen deficiency | N |
The cause: Nitrogen deficiency can be caused by under-fertilization, nutrient imbalance or excessive leaching.
General growth retardation.
Correction : Determine the cause and correct it. This may mean adding more nitrogen to the nutrient solutions. It may also mean there is too much of an antagonistic nutrient in the nutrient solution.
Detection: Nutrient analysis and/or perform plant analysis.
The youngest leaves have difficulty unfolding. The youngest leaves curl up and wilt. Necrosis at the youngest growing points and the leaf margins of the youngest leaves.
| Manganese deficiency | Mn |
With a magnesium deficiency, these green stripes around the veins are wider and the finest leaf veins also turn yellow.
| Molybdenum deficiency | Mo |
| Phosphorus deficiency | P |
| Zinc deficiency | Zn |
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

 Hoagland's solution (HS) is a hydroponic nutrient solution developed by Hoagland and Snyder in 1933. Modified by Hoagland and Arnon in 1938, and revised again by Arnon in 1950.
Hoagland's solution (HS) is a hydroponic nutrient solution developed by Hoagland and Snyder in 1933. Modified by Hoagland and Arnon in 1938, and revised again by Arnon in 1950.
It is one of the most popular standard solution compositions for growing plants in the scientific world at least with more than 21,000 citations from Google Scholar - which is not necessarily a quality aspect, see iron content of spinach.
Hoagland's solution provides all the essential elements for plant nutrition and is suitable for supporting normal growth of a wide variety of plant species. The artificial solution described by Dennis Hoagland in 1933, known as Hoagland's solution, has been modified several times, primarily by adding iron chelates and other trace elements.
In Hoagland's 1938 nutrient recipes, known as Hoagland Solutions (1 and 2), the number of trace elements was subsequently reduced to the generally recognized essential elements (B, Mn, Zn, Cu, Mo, Fe, and Cl). Later studies confirmed that their concentrations had been adjusted for optimal plant growth.
In Arnon's 1950 revision, only one concentration (Mo 0.011 ppm) was changed compared to 1938 (Mo 0.048 ppm), while the concentration of macronutrients of Hoagland solutions (0), (1), and (2) remained the same since 1933, with the exception of calcium (160 ppm) in solution. The main difference between solution (1) and solution (2) is the different use of nitrate nitrogen and the ammonium nitrogen base.
Composition and concentrations of macronutrients
in Hoagland's solutions (0, 1, 2) and in Knop's solution
| Macronutrients | Hoagland's solution 0 and 1 | Hoagland's Solution 2 | Knops solution |
| Amount in the solution | |||
| μmol/L | μmol/L | μmol/L | |
| K+ | 6,000 | 6,000 | 4,310 |
| Ca2+ | 5,000* | 4,000** | 6,094 |
| Mg2+ | 2,000 | 2,000 | 1,014 |
| NO3 − | 15,000 | 14,000 | 14,661 |
| NH4+ | - | 1,000 | - |
| SO42− | 2,000 | 2,000 | 1,014 |
| PO43− | 1,000 | 1,000 | 1,837 |
Applications:
Plant nutrients are normally absorbed into the soil solution. Hoagland's solution, originally designed to mimic a (nutrient-rich) soil solution, has high concentrations of N and K, making it ideal for developing large plants such as tomatoes and peppers.
Components
Salts, acids and complex ions are used to prepare Hoagland hydroponic solution formulations (1) and (2).
- Potassium nitrate, KNO3; Calcium
Nitrate tetrahydrate, Ca(NO3)2• 4H2O.
Magnesium sulfate heptahydrate, MgSO4• 7H2O.
Potassium dihydrogen phosphate, KH2PO4 or
Ammonium dihydrogen phosphate (NH4) H2PO4
Boric acid, H.3BO3
Manganese chloride tetrahydrate, MnCl2• 4H2O.
Zinc sulfate heptahydrate, ZnsO4• 7H2O.
Copper sulfate pentahydrate, CuSO4• 5H2O.
Molybdic acid monohydrate, H.2MoO4•H2O or
Sodium molybdate dihydrate, Na2MoO4• 2H2O.
Ferrous tartrate or iron(III)-EDTA− or iron chelate (Fe-EDDHA−)
Components for Hoagland Solution 1
To prepare the stock solutions and obtain a complete Hoagland solution, here are the ingredients:
Table 1
| Component | Amounts in solution | |
|---|---|---|
| g / l | ml / l | |
| Macronutrients | ||
| 2M KNO3 | 202 | 2.5 |
| 2M Ca (NO3)2• 4H2O. | 472 | 2.5 |
| 2M MgSO4• 7H2O. | 493 | 1 |
| 1M KH2PO4 | 136 | 1 |
| Micronutrients | ||
| H3 BO3 | 2.86 | 1 |
| MnCl2 • 4H2O | 1.81 | 1 |
| ZnsO4 • 7H2O | 0.22 | 1 |
| CuSO4 • 5H2O | 0.08 | 1 |
| H2MoO4 •H2O or | 0.09 | 1 |
| Na2MoO4 • 2H2O | 0.12 | 1 |
| iron | ||
| C12H12Fe2O18 or Sprint 138 iron chelate* * |
5 15 |
1 1.5 |
Components for Hoagland Solution 2
To prepare the stock solutions and obtain a complete Hoagland solution, here are the ingredients:
Table 2
| component | Amounts in solution | |
|---|---|---|
| g / l | ml / l | |
| Macronutrients | ||
| 2M KNO 3 | 202 | 3 |
| 2M Ca (NO3)2• 4H2O. | 472 | 2 |
| 2M MgSO4• 7H2O. | 493 | 1 |
| 1M NH4H.2PO4 | 115 | 1 |
| Micronutrients | ||
| H3BO3 | 2.86 | 1 |
| MnCl2• 4H2O | 1.81 | 1 |
| ZnsO4• 7H2O | 0.22 | 1 |
| CuSO4• 5H2O | 0.08 | 1 |
| H2MoO4•H2O. | 0.02 | 1 |
| iron | ||
|
C12H12Fe2O18, or Sprint 138 Iron Chelate * |
5 15 |
1 1.5 |
Trace element supplement according to DR Hoagland
- One liter of prepared Hoagland solution (supplemental nutrient solution, solution A) contains:
55 mg Al 2 (SO 4 ) 2
28 mg KJ
28 mg KBr
55 mg TiO 2
28 mg SnCl 2 · 2 H 2 O
28 mg LiCl
389 mg MnCl 2 · 4 H 2 O
614 mg B(OH) 3
55 mg ZnSO 4
55 mg CuSO 4 · 5 H 2 O
59 mg NiSO 4 · 7 H 2 O
55 mg Co(NO 3 ) 2 · 6 H 2 O
Source among others: https://en.wikipedia.org/wiki/Hoagland_solution
Image: Dennis Robert Hoagland - Smithsonian Libraries and Archives, Public Domain, https://commons.wikimedia.org/w/index.php?curid=159882668
ID: 717
Context:
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

 Murashige and Skoog medium (or MSO or MS0 (MS-zero) ) is the most popular plant growth medium used in laboratories worldwide for cultivating plant cell cultures on agar . MS0 was invented in 1962 by plant scientists Toshio Murashige and Folke K. Skoog during Murashige's search for a new growth regulator.
Murashige and Skoog medium (or MSO or MS0 (MS-zero) ) is the most popular plant growth medium used in laboratories worldwide for cultivating plant cell cultures on agar . MS0 was invented in 1962 by plant scientists Toshio Murashige and Folke K. Skoog during Murashige's search for a new growth regulator.
A number after the letters MS indicates the sucrose content of the medium. For example, MS0 contains no sucrose, while MS20 contains 20 g/L sucrose. Together with its modifications, it is the most commonly used medium for plant tissue culture experiments in the laboratory.
As Skoog's doctoral student, Murashige originally set out to search for a previously undiscovered growth hormone in tobacco sap. However, no such compound was found; instead, analyses of tobacco sap and tobacco ash revealed higher concentrations of certain minerals in plant tissue than previously known. A series of experiments showed that different concentrations of these inorganic nutrients significantly enhanced growth compared to existing formulations. Nitrogen, in particular, promoted tobacco growth in tissue cultures.
However, according to recent scientific findings, MS medium is not suitable as a nutrient solution for deep water culture or hydroponics , and organic compounds are not required for normal plant nutrition.
Important salts (macronutrients)
- Ammonium nitrate (NH 4 NO 3 ) 1650 mg/l
- Calcium chloride (CaCl 2 · 2H 2 O) 440 mg/l
- Magnesium sulfate (MgSO 4 7H 2 O) 180.7 mg/l
- Monopotassium phosphate (KH 2 PO 4 ) 170 mg/l
- Potassium nitrate (KNO 3 ) 1900 mg/l
Trace salts (micronutrients)
- Boric acid (H 3 BO 3 ) 6.2 mg/l
- Cobalt chloride (CoCl 2 6H 2 O) 0.025 mg/l
- Iron sulfate (FeSO 4 7H 2 O) 27.8 mg/l
- Manganese(II) sulfate (MnSO 4 · 4H 2 O) 22.3 mg/l
- Potassium iodide (KI) 0.83 mg/l
- Sodium molybdate (Na 2 MoO 4 · 2H 2 O) 0.25 mg/l
- Zinc sulfate (ZnSO 4 ·7H 2 O) 8.6 mg/l
- Ethylenediaminetetraacetic acid iron(III) sodium (FeNaEDTA) 36.70 mg/L
- Copper sulfate (CuSO 4 5H 2 O) 0.025 mg/l
Vitamins and organic compounds
- Myo-Inositol 100 mg/l
- Nicotinic acid 0.5 mg/l
- Pyridoxine HCl 0.5 mg/l
- Thiamine HCl 0.1 mg/l
- Glycine 2 mg/l
- Tryptone 1 g/l (optional)
- Indoleacetic acid 1-30 mg/l (optional)
- Kinetin 0.04–10 mg/l (optional)
Sources: https://en.wikipedia.org/wiki/Murashige_and_Skoog_medium, https://en.wikipedia.org/wiki/Toshio_Murashige
Image: By Juandev - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=47459533
ID: 701
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

Structured overview of deficiency symptoms🟩 Leaves🔻 Leaf loss & death🌕 Chlorosis (yellowing)a) Entire plant / large area
b) Intercostal / intervenal chlorosisc) Specific at high EC🍂 Necrosis / Dying Tissues🔀 Deformations and structural symptoms🌿 Shoot / growth habit⬇️ Growth problems🌸 Flowers/fruits🌼 Flower problems🍅 Fruit problems🌱 Roots |
Deficiency symptoms by elementSymptoms of lack of Al
Symptoms of lack of B
Symptoms of lack of Be
Symptoms of lack of Ca
Symptoms of lack of Cl
Symptoms of Cu deficiency
Symptoms of lack of Fe
Symptoms of lack of K
Symptoms of lack of Li
Symptoms of lack of Mg
Symptoms of lack of Mn
Symptoms of lack of Mo
Symptoms of lack of N
Symptoms of lack of Na
Symptoms of lack of NH₄⁺
Symptoms of lack of NO₃⁻
Symptoms of P deficiency
Symptoms of lack of S
Symptoms of lack of Se
Symptoms of lack of Si
Symptoms of lack of Zn
|
Possible nutrient deficiencies (sorted by probability)
| Element | Score | Probability (%) | Rating: Deficiency or overdose |
|---|
Contradictions and interactions
License: GNU GPLv3 (CopyLeft), author: Helmer Borgmann
ID: 724
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

One kilo costs about 30 to 49 euros and is enough for about 1500 liters of nutrient solution
| Contents | division |
|
120 grams of Masterblend 4-18-38 (about 1/2 cup and a tablespoon)
60 grams of magnesium sulfate (about 4 tablespoons)
|
Solution 1: mix with 500 ml water |
| 180 grams of calcium nitrate (about 3/4 cup) | Solution 2: mix with 500 ml water |
| Plant | concentration |
| Fruit-bearing bedding plants |
Solution 1: 3 ml per liter of water: for 10 liters take 30 ml, for 1 gallon = 12 ml
Solution 2: 3 ml per liter of water: for 10 liters take 30 ml, for 1 gallon = 12 ml
|
| Green leafy vegetables | Solution 1: 2.5 ml per liter of water: for 10 liters take 25 ml, for 1 gallon = 8 ml
Solution 2: 2.5 ml per liter of water: for 10 liters take 25 ml, for 1 gallon = 8 ml
|
* ) Conversion
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

The most common solutions for plant nutrition and plant tissue cultivation today are the formulations from Hoagland / Arnon (1938, 1950) and Murashige / Skoog (1962).
Hoagland and Arnon's basic formulas are replicated in liquid form by many manufacturers and sold as fertilizers to plant breeders, farmers, and consumers. Even the names Hoagland, Knop, Murashige, and Skoog are used as trademarks. Examples include Hoagland's No. 2 Basal Salt Mixture and Murashige and Skoog Basal Salt Mixture.
Hoagland and many other plant nutritionists used over 150 different nutrient solution recipes during their careers. In fact, several nutrient recipes refer to a standard name, even though they have little connection to the original formula. Several recipes were published under the name "Hoagland," and to this day, confusion persists due to the creator's memory loss regarding the original composition. You can find some of the compositions in our nutrient calculator. It's also available for download here for offline use.
Hewitt's Table 30A
Composition of selected standard nutrient solutions, modified from Hewitt (Table 30A). Concentration of elements in ppm (mg/liter).
| Reference | Ca | Mg | Na | K | B | Mn | Cu | Zn | Mo | Fe | Cl | N | P | S | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sachs (1860) | 266 | 48 | 95 | 386 | – | – | – | – | – | – | 145 | 139 | 78 | 177 | First published standard formula |
| Knop (1865) | 244 | 24 | – | 168 | – | – | – | – | – | – | – | 206 | 57 | 32 | Knop's solution |
| Panning (1915) | 208 | 484 | – | 562 | – | – | – | – | – | – | – | 148 | 448 | 640 | Shive's solution |
| Hoagland (1919) 1 | 200 | 99 | 12 | 284 | – | – | – | – | – | – | 18 | 158 | 44 | 123 | HS based on the soil solution |
| Hoagland (1920) | 172 | 52 | – | 190 | – | – | – | – | – | – | – | 158 | 38 | 67 | Hoagland's optimal nutrient solution |
| Hoagland & Snyder (1933) | 200 | 48.6 | – | 235 | 0.11 | 0.11 | 0.014 | 0.023 | 0.018 | 1.0 | 0.14 | 210 | 31 | 64 | Hoagland's solution (0) |
| Hoagland & Arnon (1938) | 200 | 48.6 | – | 235 | 0.50 | 0.50 | 0.02 | 0.05 | 0.048 | 1.0 | 0.65 | 210 | 31 | 64 | Hoagland's solution (1) |
| Hoagland & Arnon (1950) | 160 | 48.6 | – | 235 | 0.50 | 0.50 | 0.02 | 0.05 | 0.011 | 1.0 | 0.65 | 210 | 31 | 64 | Hoagland's solution (2) |
| Jacobson (1951) | – | – | – | 10.5 | – | – | – | – | – | 5.0 | – | – | – | 2.9 | Jacobson's solution |
| Hewitt (1952, 1966) | 160 | 36 | 31 | 156 | 0.54 | 0.55 | 0.064 | 0.065 | 0.048 | 2.8 | – | 168 | 41 | 48 | Long Ashton nutrient solution |
Hybrid nutrient solutions
Hybrid nutrient solutions, which consist, for example, of macronutrients from a modified Hoagland solution , micronutrients from a modified Long-Ashton nutrient solution and iron from a modified Jacobson solution , combine the physiological properties of different standard solutions into a balanced nutrient solution that enables optimal plant growth when diluted to 1⁄3 of the complete hybrid solution (cf. Nagel's Table S4, below).
Nagel's Table S4
Composition of a hybrid nutrient solution modified according to Nagel et al. Element concentrations in ppm (mg/liter).
| Reference | Ca | Mg | Na | K | B | Mn | Cu | Zn | Mo | Fe | Cl | N | P | S | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nagel et al. (2020) | 200 | 48.6 | 0.023 | 246 | 0.54 | 0.55 | 0.064 | 0.065 | 0.048 | 5.0 | 0.71 | 210 | 31 | 67 | Hybrid nutrient solution |
Source: https://en.wikipedia.org/wiki/Dennis_Robert_Hoagland#Hewitt's_Table_30A
ID: 716
Context:
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:

 Fertilizer for tomatoes: A recommendation for adaptation, depending on the growth phase
Fertilizer for tomatoes: A recommendation for adaptation, depending on the growth phase
The classic Long Ashton nutrient solution (Hewitt, 1966) is intended as a general-purpose formulation for many plant species and is therefore not optimal for high-yielding fruit vegetables such as tomatoes (Solanum lycopersicum). Tomatoes have different nutrient requirements at different stages of development, especially for N, K, Ca, Mg, and P. This information is also directly available in our fertilizer calculator .
Growth phase (vegetative, approx. up to 4th-5th leaf axis):
- Nitrogen (N): increase to approx. 10–12 mmol/L
- Potassium (K): approx. 4–5 mmol/L
- Calcium (Ca): to 3–4 mmol/L
- Magnesium (Mg): approx. 1–1.5 mmol/L
- Phosphorus (P): approx. 1–1.5 mmol/L
- Iron (Fe): to 0.05–0.1 mmol/L , e.g., by Fe-DTPA
- Sulfur (S): approx. 1–1.5 mmol/L
Flowering phase (beginning of flowering to fruit set):
- Increase N slightly (12–14 mmol/L)
- Increase K significantly (6–8 mmol/L)
- Increase P slightly (1.5–2 mmol/L)
- Ca stable at 4 mmol/L
- Mg to 1.5 mmol/L
- Keep Fe constant
Fruit/harvest phase:
- K to 8–9 mmol/L , for fruit quality
- Ca at 4–5 mmol/L , for blossom end rot prevention
- Mg at 1.5–2 mmol/L
- P constant
- Reduce N slightly (to approx. 12 mmol/L) – promotes generative growth
- S & Fe constant
Image: Peas, cabbage, beans, corn, tomato, radish, and onion. Vick's Garden and Floral Guide (1894), PD by https://openverse.org/
ID: 722
- Details
- Parent Category: Technology
- Category: Nutrient Solutions
-
Also available:



